Ceramic materials have been applied to various aspects of our lives. Ceramic materials are a class of inorganic non-metal materials made of natural or synthetic compounds after being shaped and sintered at high temperatures. It has the advantages of high melting point, high hardness, high abrasion resistance, oxidation resistance, etc.. It can be used as structural materials, tool materials, etc. Since ceramic also has some unique properties, it can also be used as a functional material. With the progress of society, people have higher and higher requirements for materials, and this performance is not only reflected in the field of science but also the area of people's lives. The progress of materials has mostly promoted the development of society, which in turn has helped the growth of materials science. In the case of ceramic materials, ceramic materials have penetrated the history of human development. They have continued to develop with the progress of history, temporarily emerging in the field of materials science.

Ceramic materials can be divided into ordinary ceramic materials and unique ceramic materials. Everyday ceramic materials are sintered from natural materials such as feldspar, clay, and quartz, and are conventional silicate materials. Ordinary ceramics have rich sources, low cost, and mature technology. This ceramic material can be divided into daily-use ceramics, building ceramics, electrical insulation ceramics, and chemical ceramics according to their performance characteristics and uses. Unique ceramic materials are made of high-purity synthetic raw materials, formed by precise control technology and sintered, and generally, have specific unique properties to meet various needs. According to their raw material components, they can be divided into carbide ceramics, oxide ceramics, nitride ceramics, and cermets. Unique ceramics have select mechanical, acoustic, optical, electrical, magnetic, thermal, and other properties, and they are also widely used.
Let us focus on the carbide ceramic materials. Carbide ceramics are ceramics containing carbon, poorly soluble compounds as the main component. One is a metal-like carbide such as titanium carbide, zirconium carbide, tungsten carbide, etc .; one is a non-metal carbide, such as carbon tetraboride, silicon carbide, and the like. Carbide is a high-temperature resistant material. Many of these carbides have melting points above 3000 ° C. Most carbides are more resistant to oxidation than carbon and graphite. Many carbides have high hardness and excellent chemical stability. Carbide ceramics also have unique properties such as high heat resistance and high hardness. Carbide ceramics are widely used as heat-resistant materials and super hard tools.

Among the high-temperature carbide structure, ceramic materials, silicon carbide ceramics, boron carbide ceramics, and titanium carbide ceramics are the three most important materials and are used most widely.
1. Silicon carbide has a diamond crystal structure and has strong covalent bonds. Silicon carbide ceramic has high hardness, good strength, high thermal conductivity, and excellent oxidation resistance. It can be used in high temperature, high pressure, strong acid, strong alkali, and high-temperature oxidation environments. It is widely used in the petroleum industry, chemical industry, and energy industry. It is also used in the fields of machinery, mining, papermaking, steelmaking, nuclear sector, microelectronics industry, and lasers.
2. The hardness of boron carbide is second only to diamond and cubic boron carbide in nature. In particular, the nearly constant high-temperature hardness is unmatched by other materials, so it has become an essential member of the superhard material family. Boron carbide has a high melting point, high hardness, high modulus, small capacity, abrasion resistance, acid, and alkali corrosion resistance, etc., and has good neutron and oxygen absorption capabilities, has a lower coefficient of expansion, excellent thermoelectric properties, is A relevant structural ceramic material. Boron carbide ceramic is an advanced excellent grinding material, which can be used for grinding, grinding, drilling, and polishing of hard materials such as gems, ceramics, tools, bearings, hard alloys, etc. Boron carbide ceramics are the first choice for industrial ceramic materials, Widely used in sandblasting machinery, electronics, information, aerospace, automotive and other industries; boron carbide ceramics have large thermal neutron capture cross-sections, have excellent neutron absorption and radiation resistance, and can be used as shielding and control materials Is the safety guarantee of the nuclear industry; boron carbide ceramics have high strength and small specific gravity, and are particularly suitable for use in bullet-proof armor, such as the protection of aircraft, vehicles, ships, and human bodies; boron carbide ceramics have the characteristics of anti-oxidation and high-temperature resistance. As advanced shaped and amorphous refractories are widely used in metallurgical fields; boron carbide ceramics have stable chemical properties, do not react with acids and alkalis, and have high synthetic positions, so they are widely used in the production of other boron-containing materials, such as bosonization Titanium, zirconium boride, etc .;
3. Titanium carbide ceramic has high strength, good thermal conductivity, high hardness, excellent chemical stability, no hydrolysis, good high-temperature oxidation resistance, and does not react with acids at room temperature. Titanium carbide ceramics are essential raw materials for hard alloys; transparent titanium carbide ceramics are right optical materials; porous titanium carbide ceramics can be used as high-temperature-resistant materials and used to make filters and photocatalytic materials when the porosity of titanium carbide ceramics is 50%, Great for artificial bones.
In most countries in the north, the rainy and snowy weather in winter is likely to cause snow in the road area to be frozen, so that the adhesion of the vehicle tires to the ground is reduced, the danger of driving is also increased, and the smooth traffic is also severely affected. According to statistics, 15%~30% of winter traffic accidents are related to road area snow icing. In order to solve the severe traffic safety problems caused by snowy roads, people actively explored and tried many methods.

With the development of science and technology and the continuous discovery of new materials, some scientists have boldly imagined that if the road is paved with conductive concrete, when the ice and snow pile up, as long as the road is energized and heated, it can quickly melt the ice and snow and solve the problem of road slippery. Keep traffic safe and smooth.
In 1980, some developed countries began to explore the feasibility of conductive concrete and studied its preparation technology. In the 1970s, the United States and the Nordic countries introduced the research and application of conductive concrete in order to solve the problem of road and bridge concrete corrosion caused by deicing salt. In the late 1990s, the study of conductive concrete began to be gradually applied in practice. In 1998, China tried to incorporate graphite into cement paste to make indoor heating floors, which was very satisfactory. In 2003, the United States applied conductive concrete to the deck of the Roca Spur Bridge, using its thermoelectric effect to deicing snow. These research results show that conductive concrete has solid prospects, and it is extremely feasible to apply to melt ice and snow.

Graphite is a relatively easy-to-obtain inorganic material that has not only good electrical and thermal conductivity but also has excellent chemical inertness. Studies have shown that the electrical resistivity of conductive commercial concrete can vary from 10-1 to 106 Ω·cm with the change of graphite content. However, it is necessary to make the commercial concrete have good conductivity when the content is high, and the graphite powder The water demand is significant, and it is necessary to increase significantly the water consumption of the commercial concrete mixture, which will cause the strength of the commercial concrete to decrease as the amount of graphite powder increases rapidly. When the content of graphite exceeds 12%, the increase of electrical conductivity is not apparent, while the compressive strength decreases sharply with the increase of graphite content. When the amount of graphite is more than 20%, the compressive strength is less than 10MPa, and the resistivity is less than 10MPa. Less than 50 Ω·cm.

The incorporation of graphite powder makes the workability of the concrete mixture worse, but it can meet the requirements of engineering construction. As the amount of graphite powder is increased, the strength of conductive concrete decreases; the fineness of graphite powder has little effect on the strength of conductive concrete. The electrical resistivity of the conductive concrete decreases with the increase of the fineness and the amount of the graphite powder. After continuous energization, it has an excellent electrothermal effect and a power generation effect. Conductive concrete with excellent workability, strength, and electrical conductivity can be prepared by using graphite powder. The conductive concrete is mixed into ordinary concrete, and the concrete is transformed into a new composite material with specific conductivity. After the power is turned on, the concrete is energized and heated. As the temperature of the road surface rises, the snow and ice are melted by heat. Conductive concrete can melt snow in a timely and efficient manner, ensure smooth traffic and safety, reduce national economic losses caused by ice and snow disasters, be green, and meet the requirements of sustainable development. As a new type of composite functional concrete, it not only has the superior mechanical ability of ordinary concrete, but also has good electrical conductivity and thermal conductivity; it can effectively avoid the traffic accident risk caused by snow and ice in winter road area and can solve the deicing salt. The economic and environmental problems brought about have good application prospects.

Black graphite can be transformed into expensive ornate diamonds. Is this transformation a physical or chemical change? We can answer this question from the analysis of the following aspects.

1. Analysis from the characteristics of chemical changes
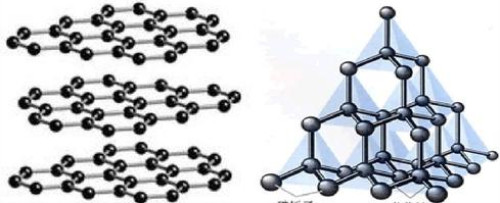
We know that chemical changes are often called chemical reactions. Chemical shifts are the formation of other substances when they change. Materials are transformed into new elements of entirely different nature through chemical reactions, which are characteristic of chemical changes. When graphite, a pure substance composed of carbon, becomes diamond under certain conditions, although diamond is also a pure substance composed of carbon, the properties of diamond are quite different from those of graphite (the chemical properties of graphite are more active than diamond); Another element of carbon. This shows that the diamond is a new substance that turns from graphite during the change. Changes in the formation of new materials are not physical changes.
2. From the perspective of crystal structure
When graphite is converted into diamond, the graphite crystal structure is destroyed, the weak bonding force between the graphite layer and the layer is broken or changed, or the chemical bond and bonding mode between the carbon atoms in the hexagonal plane is also significantly improved. The combination between them is regularly combined into a vertical structure according to the form and requirements of the diamond. That is, the layered structure of graphite is transformed into a regular tetrahedral structure of diamond. According to the fact that the same substance has only one structure, graphite and diamond are two materials with different crystal structures. Since the process of change changes from one element to another, it is not a physical change. It can be seen that the transformation of matter from one structure to another is a chemical change process.
3. Analysis from the perspective of thermal effects
This transition is an exothermic reaction. The form in which energy is converted into heat during a chemical reaction during this transition is illustrated. When graphite turns into a diamond, it is a chemical reaction that absorbs heat. Therefore, the conversion of graphite to diamond under certain conditions is not a physical change.
4. From the perspective of catalyst
The transformation of graphite into diamond must be carried out under high temperature and high pressure. Even at a temperature of 2000 ° C to 4000 ° C and a weight of 60,000 to 120,000 atmospheres, the transformation rate is still low, and chromium, iron, and platinum are required as catalysts. Depending on the principle that the enzyme can only change the price of the chemical reaction, the catalyst cannot change the rate of physical change. When graphite is converted to diamond, the catalyst is used to accelerate the reaction rate. If this shift is a material change, does it make sense to use the catalyst? It can be seen that this change is not physical.

In summary, the mutual transformation of diamond and graphite under certain conditions is a chemical change. The mutual transformation of allomorphs under certain conditions is a chemical change. Some people say that the mutual transformation of diamond and graphite under certain conditions is a physical change process. This is not true. A shift in energy often accompanies a chemical reaction. This energy change can be expressed in the form of light energy, electrical energy, mechanical energy, or thermal energy. It is often shown in the form of heat, sometimes releasing heat and sometimes absorbing heat.
Similar to lead-based and nickel-based batteries, lithium ions use a positive electrode (cathode), a negative electrode (anode), and an electrolyte as a conductor. The positive wire is a metal oxide, and the negative electrode is composed of porous graphite. During discharge, lithium ions move from the negative electrode to the positive electrode through the electrolyte and the separator; during charging, lithium ions flow from the positive electrode to the negative electrode in opposite directions.

When the battery is charged and discharged, Li+ shuttles between the positive and negative electrodes, during discharge, the anode oxidizes, loses electrons, and the cathode is reduced to obtain particles; when charging, the charge moves in the opposite direction.
There are many types of lithium-ion batteries, depending on the electrode material. But when you choose different materials, the battery performance will vary greatly.
The positive electrode materials all contain Li+. Common lithium cobalt oxide (lithium cobalt oxide), lithium manganese oxide (also known as spinel or lithium manganate), lithium iron phosphate, nickel cobalt manganese ternary material (NMC) [3] and lithium nickel cobalt Aluminum oxide (NCA). All of these materials have a theoretical upper energy limit (lithium-ion has a theoretical capacity of about 2000 kWh, which is more than ten times the specific energy of a commercial lithium-ion battery).
Sony's original lithium-ion battery uses coke (a coal product) as a harmful electrode material. Since 1997, most lithium-ion battery manufacturers, including Sony, have converted anode materials to graphite, resulting in a flat discharge curve. Graphite is a form of carbon that is used in pencils. It can store lithium ions well during charging and has a long cycle and excellent stability. Of the carbon materials, graphite is the most common, followed by hard carbon and soft carbon. Other carbons, such as carbon nanotubes, have not yet found their commercial use. The figure below compares the voltage discharge curves of a modern lithium-ion battery with graphite as the negative electrode and a lithium-ion battery with an old coke negative electrode.

In the normal operating discharge range, the battery should have a flat voltage curve, which is better than the former coke.
Anode materials are also evolving, and researchers are continually experimenting with new materials, including silicon-based alloys. In this alloy, six carbon atoms are bonded to one lithium-ion, and one silicon atom can bond four lithium ions. This means that the negative silicon electrode can theoretically store ten times the energy of the graphite material. At present, silicon materials have increased by 20%-30% in specific capacity at the cost of reducing load potential and cycle life. However, the problem is that during the charging process, lithium ions are easily expanded in volume after being embedded in the silicon-based material (growing to more than four times the initial size).
The nanostructured lithium titanate salt has good cycle life and load capacity, excellent low-temperature performance, and functional safety performance as a harmful electrode material. Still, its specific function is low, and the cost is high.
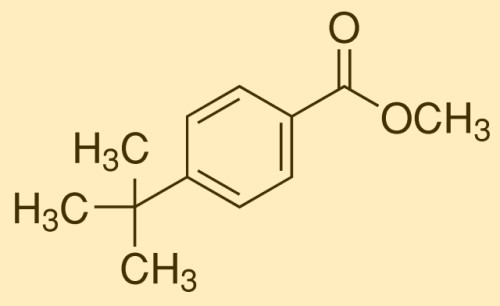
P-test-butyl benzoic acid (PTBBA) is a colorless needle crystal or crystalline powder. It is an important organic synthesis intermediate widely used in chemical synthesis, cosmetics, flavors, and fragrances, etc., such as alkyd resin. Improvers, cutting oils, lubricant additives, food preservatives, stabilizers for polyethylene.

The synthesis method of p-test-butyl benzoic acid, at present, the existing synthesis method is oxidative synthesis, mainly including solventless oxidation method, nitric acid oxidation method, liquid-phase catalytic oxidation method, potassium permanganate oxidation method, microwave synthesis method High-temperature gas phase oxidation method. These methods are all based on p-test-but toluene.
Use of p-test-butyl benzoic acid:
P-test-butyl benzoic acid is an important organic synthesis intermediate. Its main applications are:
1. Used as a modifier for the production of alkyd resins;
2. Used as cutting oil and lubricating oil additive; used as polypropylene nucleating agent;
3. Used as a food preservative;
4, p-tert-butyl benzoic acid can be used as a regulator of polyester polymerization;
5. The phosphonium salt, sodium salt, zinc salt and the like of p-test-butyl benzoic acid can be used as a stabilizer for polyethylene;
6, p-tert-butyl benzoic acid can also be used as an additive for automotive deodorants, an outer film of oral drugs, alloy preservatives, lubricating additives, polypropylene nucleating agents, polyvinyl chloride heat stabilizers, metalworking cutting fluids, anti- Oxygen agents, alkyd resin improvers, fluxes, dyes, and new sunscreens;
7. P-test-butyl benzoic acid is also used in the production of methyl p-test-butyl benzoate, which is widely used in chemical synthesis, cosmetics, flavors, and fragrances.
Storage of p-test-butyl benzoic acid:
Storehouses storing p-test-butyl benzoic acid should be kept ventilated and dried at low temperatures; they must be stored separately from strong oxidants and strong bases.
First-aid measures using p-test-butyl benzoic acid:
1. This product is moderately toxic. The oral LD50 of rats is 568 mg/kg.
2. If inhaled, move the patient to fresh air. If breathing stops, perform artificial respiration. Seek medical attention promptly.
3. In case of skin contact, rinse with soap and plenty of water. Immediately take the patient to the hospital. Seek medical attention quickly.
4. In case of eye contact, rinse thoroughly with plenty of water for at least 15 minutes and seek medical advice immediately.
5. If you misuse it, don't give anything to the unconscious person through the mouth. Rinse with water. Seek medical attention immediately.

Potential health effects of p-test-butyl benzoic acid:
1. Inhalation of p-test-butyl benzoic acid is harmful to health and may cause respiratory irritation.
2, swallowing and swallowing p-tert-butyl benzoic acid is detrimental to the human body.
3, p-tert-butyl benzoic acid contact with the skin if absorbed by the skin will be toxic, may cause skin irritation.
4. P-test-butyl benzoic acid causes eye irritation.




