Professional and high-quality metal alloys, ceramic products and concrete additives | RBOSCHCO
PRODUCT PARAMETERS
Description
Overview of Manganese Dioxide MnO2
Manganese Dioxide(MnO2) is an inorganic compound belonging to the oxide of manganese. It usually exists in the form of minerals in nature, such as pyrolusite. MnO2 is a black or brown solid powder with the characteristic of being insoluble in water. Its crystal structure is diverse, including α- MnO2 β- MnO2 and γ- MnO2, etc., exhibit unique physical and chemical properties due to their different crystal structures.
MnO2 is known for its strong oxidizing properties and can participate in various redox reactions. It remains stable at high temperatures and has a high melting point and high specific surface area. In addition, MnO2 also exhibits good chemical stability and is usually less likely to react with other substances except in certain strong acidic environments.

(Manganese Dioxide MnO2 CAS 1313-13-9)
Features of Manganese Dioxide MnO2
Strong oxidizing properties: MnO2 has strong oxidizing properties and can participate in various redox reactions, transforming into lower valence manganese compounds.
Insoluble in water: MnO2 is a solid that is insoluble in water, which allows it to remain stable in certain applications.
Multiple crystal structures: MnO2 has multiple crystal structures, such as α- MnO2 β- MnO2 and γ- MnO2 and other crystal structures endow it with different physical and chemical properties.
High melting point: MnO2 has a high melting point, which allows it to maintain its structural stability even in high temperature environments.
Good chemical stability: Except in strong acidic environments, MnO2 usually exhibits good chemical stability and is not prone to chemical reactions with other substances.
High specific surface area: MnO2 can be prepared into nanoscale powders with high specific surface area, which makes it have broad application prospects in catalysis, electrochemistry, and other fields.
Parameter table of Manganese Dioxide MnO2
| Manganese Dioxide Properties | |
| Other Names | manganese oxide, MnO2 powder |
| CAS No. | 1313-13-9 |
| Compound Formula | MnO2 |
| Molecular Weight | 86.94 |
| Appearance | Black Powder |
| Melting Point | 535 °C |
| Boiling Point | N/A |
| Density | 5.03 g/cm3 |
| Solubility in H2O | Insoluble |
| Exact Mass | 86.9279 |
| Manganese Dioxide Health & Safety Information | |
| Signal Word | Warning |
| Hazard Statements | H302 + H332 |
| Hazard Codes | Xn, O |
| Risk Codes | N/A |
| Safety Statements | N/A |
| Transport Information | NONH |
Application of Manganese Dioxide MnO2
Battery industry: MnO2 is an important positive electrode material for alkaline zinc manganese batteries and lithium-ion batteries, providing the energy source required for batteries.
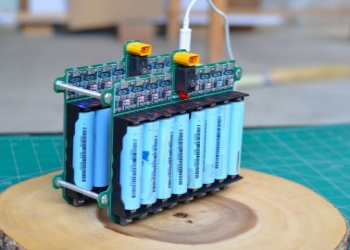
Application of Manganese Dioxide MnO2
Catalyst: Due to the strong oxidizing properties and high specific surface area of MnO2, it is often used as a catalyst for various chemical reactions, such as oxidation, reduction, and decomposition reactions.
Water treatment: MnO2 can be used to remove organic pollutants, heavy metal ions, and other harmful substances in the water treatment process, improving water quality.
Pigments and coatings: MnO2 has good coloring performance and stability, and is therefore used as an additive in pigments and coatings, giving products a deep black or brown color.

Application of Manganese Dioxide MnO2
Electrochemical field: MnO2 has a wide range of applications in the electrochemical field, such as supercapacitors, lithium-ion batteries, and solar cells, to improve energy storage and conversion efficiency.
Ceramic and glass industry: MnO2 can be used as a colorant and flux in the ceramic and glass industry, improving the appearance and performance of products.

Application of Manganese Dioxide MnO2
Production Method of Manganese Dioxide MnO2
Pyrolusite Ore Method
The pyrolusite ore is crushed and ground, then leached with sulfuric acid and an appropriate reducing agent like iron(II) sulfate to convert MnO2 to manganese sulfate. The solution is purified and then electrolyzed to deposit MnO2 on the anode.
Chemical Oxidation Method
Manganese sulfate solution is oxidized by an oxidant such as potassium permanganate KMnO4 or sodium chlorate NaClO3 in an alkaline or neutral medium.
Thermal Decomposition Method
Some manganese compounds like manganese nitrate MnNO32 or manganese carbonate MnCO3 are heated. MnNO32 decomposes at high temperatures to form MnO2, nitrogen dioxide and oxygen.

Company Profile
RBOSCHCO is a trusted global chemical material supplier & manufacturer with over 12-year-experience in providing super high-quality chemicals and nanomaterials, including boride powder, nitride powder, graphite powder, sulfide powder, 3D printing powder, etc.
The company has a professional technical department and Quality Supervision Department, a well-equipped laboratory, and equipped with advanced testing equipment and after-sales customer service center.
If you are looking for high-quality Manganese Dioxide MnO2, please feel free to contact us or click on the needed products to send an inquiry.
Storage Condition of Manganese Dioxide MnO2
Store in a cool and dry area, ideally at a temperature between 15°C and 25°C, and avoid high humidity to prevent agglomeration and influence on its properties.
Use airtight packaging to isolate it from the air and reduce the possibility of reactions with gases such as oxygen and carbon dioxide in the air.
Keep it away from flammable and explosive substances, as (MnO_2) is an oxidizing agent and can react with combustible materials, posing a safety hazard. Also, separate it from reducing agents to avoid redox reactions that alter its properties.
Payment Term
L/C, T/T, Western Union, Paypal, Credit Card etc.

Shipment Term
By sea, by air, by express, as customers request.
5 FAQs of Manganese Dioxide MnO2
Q1
What role does Manganese Dioxide MnO2 typically play in chemical reactions?
Answer : Manganese Dioxide MnO2 is commonly used as a catalyst or oxidant in chemical reactions. Due to its strong oxidizing properties, it can accelerate the rate of many chemical reactions and may be reduced during the reaction. For example, in batteries, MnO2 can serve as a positive electrode material, participating in redox reactions and releasing electrical energy.
Q2
How to prepare Manganese Dioxide MnO2?
Answer : There are various methods for preparing Manganese Dioxide MnO2, and one common method is to react manganese salts (such as manganese sulfate) with alkaline substances (such as sodium hydroxide) to generate manganese hydroxide precipitates, which are then oxidized and heat treated to ultimately convert into MnO2. During the preparation process, it is necessary to control reaction conditions such as temperature, pH value, and reaction time to obtain high-purity and well crystallized MnO2.
Q3
In which fields does Manganese Dioxide MnO2 have practical applications?
Answer : Manganese Dioxide MnO2 has practical applications in multiple fields. In the battery industry, it is widely used as a positive electrode material for dry batteries, such as alkaline zinc manganese batteries and lithium-ion batteries. In addition, MnO2 is also used to manufacture catalysts, pigments, ceramics, and glass. In the field of environmental protection, MnO2 is also used for water treatment to remove harmful substances from water.
Q4
What are the physical properties of Manganese Dioxide MnO2?
Answer : Manganese Dioxide MnO2 is a black or brown solid powder with the characteristic of being insoluble in water but soluble in acid. Its crystal structure is diverse, and common crystal forms include α- MnO2 β- MnO2 and γ- MnO2, etc. In addition, MnO2 has a high melting point and density, as well as good chemical stability.
Q5
How to determine the purity of Manganese Dioxide MnO2?
Answer : The purity of Manganese Dioxide MnO2 can usually be determined through chemical analysis, thermogravimetric analysis, X-ray diffraction, and other methods. Chemical analysis can determine the content of manganese and other elements in the sample, thereby calculating the purity of MnO2. Thermogravimetric evaluation can measure the high quality modifications of examples during heating to assess the presence of pollutants. X-ray diffraction can evaluate the crystal framework of the sample and identify the visibility of various other pollutant stages. By integrating the outcomes of these methods, the purity of MnO2 can be accurately assessed.
REQUEST A QUOTE
RELATED PRODUCTS

Solid Silica Dispersant TRMIBKSiOT10 High Quality Silica Nanoparticle Dispersion

Cas 7446-07-3 99.99% 99.999% Tellurium Dioxide ( Oxide ) TeO2 Powder Price

Bismuth Oxide Powder Bi2O3 Powder Price

Multi-purpose 99% Fe3O4 Super Fine High Purity Fe Magnetite Iron Powder

High Purity 99.9% Antimony Doped Tin Oxide/ATO Nano Powder CAS 128221-48-7







