Professional and high-quality metal alloys, ceramic products and concrete additives | RBOSCHCO
Metallic copper

High Quality 99.9% C11000 Copper Bar Factory Price

Billow Hot Selling C11000 C1100 99.95% Pure Busbar Copper Rod Square Flat Round Brass Copper Bars

Sticks Phosphor Copper Brazing Alloy Welding Rods BCup-2
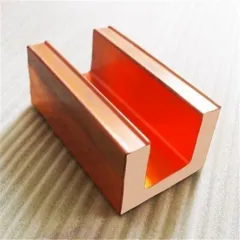
Extruded Copper Profiles

Factory Wholesale Earth Rod Hardware with Good Quality Copper Pole Line Hardware

Customized Size Oxygen-Free Copper Rod Low Oxygen Content for Superior Conductivity
Metallic Copper Overview
Cu (atomic number 29) is an ancient and widely existing metal known for its red-orange metallic luster, high conductivity, thermal conductivity, and good elasticity. It is widely used in cables, electrical components, building materials, and alloys. Copper alloys such as bronze and brass perform excellently in industry. Copper is durable and recyclable and has historically been used to manufacture minerals such as chalcopyrite and turquoise. Copper participates in respiration and iron transport in the human body, containing about 0.01% of the Earth's crust. It has a melting point of 1083 ℃ and a boiling point of 2567 ℃.
Specifications of Metallic Copper
| Parameter | Description |
|---|---|
| Material Name | Metallic Copper |
| CAS Number | 7440-50-8 |
| Element Symbol | Cu |
| Purity (%) | ≥99.5% (typical; may vary based on grade) |
| Particle Size Distribution (PSD) | Average Particle Size (D50): 10 µm to 50 µm (varies by product grade) |
| Morphology | Spherical, Irregular, or Plate-like (depends on manufacturing process) |
| Surface Area (m²/g) | Varies with particle size and shape |
| Bulk Density (g/cm³) | Typically around 5.0 - 6.0 g/cm³ |
| True Density (g/cm³) | Approximately 8.96 g/cm³ |
| Tap Density (g/cm³) | Increases after tapping due to reduced air voids |
| Flowability | Measured by Hall Flow Rate or similar methods |
| Oxygen Content (%) | ≤0.05% (varies by purity grade) |
| Moisture Content (%) | ≤0.1% |
| pH Value | Neutral to slightly acidic |
| Electrical Conductivity (S/m) | Excellent conductor; typically around 5.96 × 10^7 S/m at 20°C |
| Melting Point (°C) | 1,085°C |
| Boiling Point (°C) | 2,562°C |
| Thermal Conductivity (W/m·K) | High thermal conductivity; approximately 401 W/m·K at 20°C |
| Coefficient of Thermal Expansion (CTE) (1/°C) | 16.5 x 10^-6 /°C from 20°C |
| Magnetic Properties | Non-magnetic |
| Chemical Reactivity | Stable under standard conditions; reactive with strong oxidizers |
| Solubility in Water | Insoluble |
| Safety Data | Refer to Material Safety Data Sheet (MSDS) |
| Storage Conditions | Store in a cool, dry place away from incompatible materials |
| Packaging | Typically supplied in sealed containers or bags to prevent oxidation |
Characteristic of Metallic Copper
Good and thermal conductivity: Copper is a very good conductive material, second only to silver, and is therefore widely used in cables and electrical and electronic components. Meanwhile, copper also has high thermal conductivity and is commonly used in manufacturing equipment such as radiators and heat exchangers. Sorry, the service is too hot. It would be very convenient for you to try various shapes and structures of metal products later.
Corrosion resistance: Copper has strong corrosion resistance in seawater and the atmosphere, so it is often used to manufacture various containers and pipelines in contact with corrosive media.
Good welding performance: Copper has good welding performance and can be welded with various metals, making it widely used in manufacturing.
Beautiful appearance: Copper has a golden to purple red color, which has a beautiful appearance and is often used in fields such as architecture and decoration.
Recyclability: Copper is a durable metal that can be recycled multiple times without prejudice to its mechanical properties, making it highly environmentally friendly.

Metallic Copper Application
1.Construction field: Metal copper is a high-quality building material widely popular in the construction industry due to its good corrosion resistance and beautiful appearance. It can be used to make roofs, doors and windows, sinks, pipes, etc. In addition, metal copper is also used to make indoor decorations, such as lamps and ornaments.
2.Electronic field: Due to its excellent conductivity, copper metal has a wide range of employ in the electronic field, such as in the manufacturing of cables, wires, wires, etc. In addition, copper metal is also used to make industrial appliances, electronic components, and semiconductor materials.
3.Transportation field: Metal copper also has important applications in the transportation field. For example, it can manufacture thermal and conductive components in rail transit equipment such as subways, high-speed trains, trams, etc., such as contact wires, electrical contacts, etc. In addition, metallic copper is also used in other transportation fields, such as shipbuilding and automotive manufacturing.
4.Home Furnishings: Metal copper is widely used in the manufacturing of household items, such as tableware, kettles, teapots, etc., due to its unique luster. In daily necessities, metal copper can also be made into various accessories, clocks, candlesticks, etc.
5.Petrochemical and mechanical metallurgy fields: Metal copper also has important applications, especially in environments that require high corrosion resistance, wear resistance, and thermal conductivity.
6.Medical field: Metal copper also has certain antibacterial properties, so it can be used to manufacture medical devices and food processing equipment.
Company Profile
Rboschco.com is a trusted global chemical material supplier & manufacturer with over 12-year-experience in providing super high-quality chemicals and nanomaterials, including Metallic Copper, nitride powder, graphite powder, sulfide powder, 3D printing powder, etc.
The company has a professional technical department and Quality Supervision Department, a well-equipped laboratory, and equipped with advanced testing equipment and after-sales customer service center.
If you are looking for high-quality Metallic Copper, please feel free to contact us or click on the needed products to send an inquiry.
Payment Term
L/C, T/T, Western Union, Paypal, Credit Card etc.
Shipment Term
By sea, by air, by express, as customers request.

Storage Conditions
- The storage environment should be ventilated, dry, and clean to avoid dust accumulation and contact with other metals of different materials to prevent rust and pollution. The temperature of the warehouse should be maintained at 15-30 ℃, and the relative humidity should be maintained at 40-80%.
- When storing, each layer should be placed in separate packaging, and the number of copper strips in each packaging layer should be moderate to avoid prolonged storage.
- It is not allowed to store substances such as acid, alkali, and salt in the same warehouse, especially electrolytic copper cannot be mixed with rubber and acid-sensitive materials.
- If rust is found on the copper material, it should be promptly wiped off with a burlap or copper wire brush. Don't use a steel wire brush or apply oil. Severe rust should be isolated and stored in addition to rust removal and should not be stored for a long time.
- During transportation, avoid pulling, dragging, dropping, bumping, and other behaviors to avoid damaging the copper material.
FAQ
Q1
What are the conductivity and thermal conductivity of pure copper?
Re:Pure copper is known for its excellent conductivity and thermal conductivity. In terms of conductivity, pure copper is one of the metals with the highest conductivity, second only to silver and gold. This makes pure copper ideal for manufacturing cables and electrical and electronic components, as it can efficiently transmit current without generating excessive heat. Pure copper also performs well in terms of thermal conductivity and can quickly transfer heat from one place to another. This characteristic makes pure copper crucial in thermal management applications such as radiators, heat exchangers, and heating elements.
Q2
What are the advantages of copper alloys (such as bronze and brass) compared to pure copper?
Re:Copper alloys, such as bronze (copper-tin alloy) and brass (copper-zinc alloy), have significant advantages over pure copper. Firstly, by adding other metal elements, copper alloys can improve the mechanical properties of pure copper, such as strength, hardness, and wear resistance. This makes copper alloys more advantageous in industrial applications that require higher loads or more complex working environments. Secondly, copper alloys can also optimize their electrical, thermal, and corrosion resistance properties by adjusting the types and proportions of alloying elements to meet the specific application requirements.
Q3
What factors will affect the formation and distribution of copper deposits?
Re:Various factors spill over the formation and distribution of copper deposits. Firstly, the abundance of copper elements in the crust is the fundamental condition determining the formation of copper deposits. Secondly, geological structure and magmatic activity significantly impact the formation of copper deposits. For example, geological activities such as plate collisions and volcanic eruptions can promote copper-bearing magma's ascent and cooling solidification, forming copper deposits. In addition, hydrothermal and sedimentary processes are also important mechanisms for the formation of copper deposits. Hydrothermal action refers to the process in which underground hot water carries copper elements up along faults or fractures and precipitates to form copper deposits at a certain depth on the surface or underground. Sedimentation is the process in which copper elements are transported as dissolved or particulate matter in surface or underwater environments and gradually accumulate in sediments, ultimately forming copper deposits.
Q4
How do the physical properties of copper, such as density, melting point, and boiling point, affect its application?
Re:The physical properties of copper have a significant impact on its applications. Firstly, the density of copper is moderate (8.96 grams per cubic centimeter), neither too light nor too heavy, making it an ideal material in many engineering applications. Secondly, copper has a high melting point (1083 degrees Celsius), which means it can maintain good stability in high-temperature environments and pertains to manufacturing components that must withstand high temperatures. In addition, copper has a high boiling point (2567 degrees Celsius), which allows it to maintain a longer service life during high-temperature processing and smelting. These physical properties collectively determine the widespread application of copper in fields such as cables, electrical components, building materials, and alloy manufacturing.
Q5
What are the main steps in the copper smelting process?
Re:Extracting copper-containing ores from copper mines. Crush and grind the extracted ore to reduce its particle size, making it easier for subsequent smelting operations. Using the flotation method to separate copper minerals from gangue in ores. During the flotation process, flotation agents are added to make the surface of copper mineral particles hydrophilic or hydrophobic, thereby separating them from gangue. The copper minerals from flotation are usually melted using pyrometallurgical or wet smelting methods. Fire smelting is melting copper minerals at high temperatures to separate copper from other impurities; Wet smelting uses chemical reactions to extract copper from ores. The crude copper obtained from smelting needs to be refined to improve copper's purity further. During the refining process, impurities in crude copper are removed by electrolysis or other chemical methods. The refined copper can be cast into various shapes of copper ingots or materials and further processed into copper pipes and wires.
