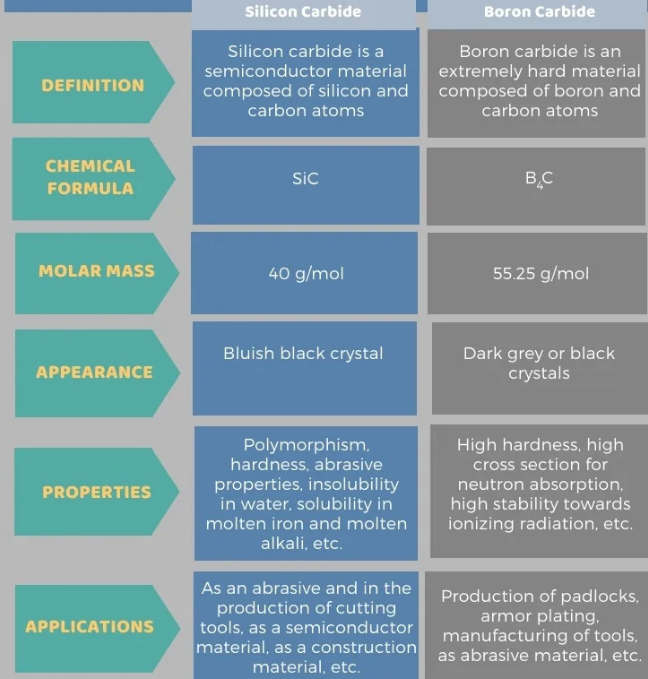
The main difference between silicon carbide and boron carbide is that in silicon carbide one silicon atom bonds to one carbon atom, whereas in boron carbide four boron atoms bond to one carbon atom.
Silicon carbide and boron carbide are carbon compounds. Both of these are very hard materials. They have different chemical and physical properties.
What is Silicon Carbide?
Silicon carbide is a semiconductor material made up of silicon and carbon atoms. The chemical formula of the compound is SiC. So, it has a silicon atom bonded to a carbon atom by a covalent bond. The material, also known as emery, is found in nature in the form of mossanite, an extremely rare mineral. Therefore, silicon carbide is mostly made as a synthetic material.
The molar mass of silicon carbide is 40g/mol. The material has a blue-black iridescence crystal structure, but is colorless in its pure form. The black color is due to the presence of iron as an impurity. Moreover, it is insoluble in water, but soluble in molten iron and lye. However, we can find about 250 crystal types of silicon carbide. This compound shows polymorphism. Here, alpha silicon carbide is the most common and stable form. It forms at very high temperatures and has a hexagonal crystal structure.
Silicon carbide has many uses. Mainly used for abrasive and cutting tool production. It is also an important structural material. Such as composite armor, ceramic coating bulletproof vests, high-temperature kilns, etc. In addition, silicon carbide is used to make car parts and as a semiconductor material.
What is Boron Carbide?
Boron carbide is an extremely hard material made of boron and carbon atoms. The chemical formula of this compound is B4C. So, it has four boron atoms bonded to one carbon atom. It is second only to diamond and cubic boron nitride in hardness. Therefore, it is also known as the "black diamond".
The molar mass of boron carbide is 55.25g/mol. It appears as a dark gray or black powder or crystal. It is insoluble in water. This material is known for its high hardness, high neutron absorption cross-section, and high stability to ionizing radiation. In addition, it has semiconductor properties. Therefore, the electronic properties of boron carbide are mainly hopping transport. In general, it is a P-type semiconductor.
Boron carbide is a synthetic material. It can be prepared by reducing boron trioxide to boron carbide in the presence of carbon. The reaction requires carbon or magnesium as a reducing agent.
What is the Difference Between Silicon Carbide and Boron Carbide?
The main difference between silicon carbide and boron carbide is that in silicon carbide one silicon atom bonds to one carbon atom, whereas in boron carbide four boron atoms bond to one carbon atom. Silicon carbide is a blue-black crystal, while boron carbide is a dark gray or black crystal.
The following infographic summarizes the differences between silicon carbide and boron carbide.
SiC and B4C Price
The price is influenced by many factors including the supply and demand in the market, industry trends, economic activity, market sentiment, and unexpected events.
If you are looking for the latest SiC and B4C price, you can send us your inquiry for a quote. (sales1@rboschco.com)
SiC and B4C Supplier
RBOSCHCO is a trusted global chemical material supplier & manufacturer with over 12-year-experience in providing super high-quality chemicals and nanomaterials. The company export to many countries including the USA, Canada, Europe, UAE, South Africa, Tanzania, Kenya, Egypt, Nigeria, Cameroon, Uganda, Turkey, Mexico, Azerbaijan, Belgium, Cyprus, Czech Republic, Brazil, Chile, Dubai, Japan, Korea, Vietnam, Thailand, Malaysia, Indonesia, Australia, Germany, France, Italy, Portugal, etc.
As a leading nanotechnology development manufacturer, RBOSCHCO dominates the market. Our professional work team provides perfect solutions to help improve the efficiency of various industries, create value, and easily cope with various challenges.
If you are looking for SiC and B4C, please send an email. (sales1@rboschco.com)




