Black graphite can be transformed into expensive ornate diamonds. Is this transformation a physical or chemical change? We can answer this question from the analysis of the following aspects.

1. Analysis from the characteristics of chemical changes
We know that chemical changes are often called chemical reactions. Chemical shifts are the formation of other substances when they change. Materials are transformed into new elements of entirely different nature through chemical reactions, which are characteristic of chemical changes. When graphite, a pure substance composed of carbon, becomes diamond under certain conditions, although diamond is also a pure substance composed of carbon, the properties of diamond are quite different from those of graphite (the chemical properties of graphite are more active than diamond); Another element of carbon. This shows that the diamond is a new substance that turns from graphite during the change. Changes in the formation of new materials are not physical changes.
2. From the perspective of crystal structure
When graphite is converted into diamond, the graphite crystal structure is destroyed, the weak bonding force between the graphite layer and the layer is broken or changed, or the chemical bond and bonding mode between the carbon atoms in the hexagonal plane is also significantly improved. The combination between them is regularly combined into a vertical structure according to the form and requirements of the diamond. That is, the layered structure of graphite is transformed into a regular tetrahedral structure of diamond. According to the fact that the same substance has only one structure, graphite and diamond are two materials with different crystal structures. Since the process of change changes from one element to another, it is not a physical change. It can be seen that the transformation of matter from one structure to another is a chemical change process.
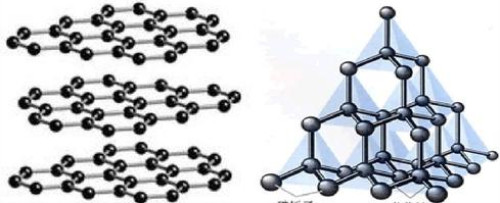
3. Analysis from the perspective of thermal effects
This transition is an exothermic reaction. The form in which energy is converted into heat during a chemical reaction during this transition is illustrated. When graphite turns into a diamond, it is a chemical reaction that absorbs heat. Therefore, the conversion of graphite to diamond under certain conditions is not a physical change.
4. From the perspective of catalyst
The transformation of graphite into diamond must be carried out under high temperature and high pressure. Even at a temperature of 2000 ° C to 4000 ° C and a weight of 60,000 to 120,000 atmospheres, the transformation rate is still low, and chromium, iron, and platinum are required as catalysts. Depending on the principle that the enzyme can only change the price of the chemical reaction, the catalyst cannot change the rate of physical change. When graphite is converted to diamond, the catalyst is used to accelerate the reaction rate. If this shift is a material change, does it make sense to use the catalyst? It can be seen that this change is not physical.

In summary, the mutual transformation of diamond and graphite under certain conditions is a chemical change. The mutual transformation of allomorphs under certain conditions is a chemical change. Some people say that the mutual transformation of diamond and graphite under certain conditions is a physical change process. This is not true. A shift in energy often accompanies a chemical reaction. This energy change can be expressed in the form of light energy, electrical energy, mechanical energy, or thermal energy. It is often shown in the form of heat, sometimes releasing heat and sometimes absorbing heat.




