High-entropy alloys (High-entropy alloys) referred to as HEA, is an alloy formed by five or more kinds of equal or approximately equal amounts of metals. Since high-entropy alloys may have many ideal properties, they have received considerable attention in materials science and engineering. In the past, the primary metal components in alloys may be only one or two. For example, it will be based on iron, and then add some trace elements to improve its characteristics, so the result is an iron-based alloy. In the past concept, if more metals are added to the alloy, the material will be embrittled, but the high-entropy fusion is different from the previous alloys. Many minerals will not be embrittled. It is new material.

With the development of high-entropy alloys, the concept of high-entropy alloys has been continuously improved; so far, the development of high-entropy composites has mainly experienced three stages. From the perspective of alloy composition elements and phase structure, the development characteristics of high-entropy alloys can be summarized as follows:
1) The first generation of high-entropy composites: composed of 5 or more alloying elements, the compositional element content ratio is an equal atomic ratio, and the phase structure is a single-phase complex alloy;
2) The second-generation high-entropy fusion: It is composed of 4 or more alloying elements. The content ratio of the constituent parts can be non-equiatomic, and the phase structure is a two-phase or multi-phase complex solid solution alloy;
3) High-entropy film or ceramic.
Materials are often prone to low-temperature brittleness. Composite materials such as high-entropy alloys exhibit unique mechanical properties at low temperatures due to the combined action of slip and twinning mechanisms. When multiple deformation mechanisms coexist, it is of considerable significance to find out how the various deformation mechanisms compete or cooperate. The CrMnFeCoNi alloy studied in this paper has a strength of 2.5GPa and a plasticity of 62% at a low temperature of 15K. The study found that stacking faults play a crucial role in bridging dislocation slippage and sawtooth. At low temperatures, various competitive mechanisms can work together to produce high strength and high ductility materials.
The mechanical behavior of materials under extreme conditions has always been a research hotspot. At high temperatures, plastic deformation is determined by atomic diffusion. Atomic diffusion will lead to the degradation of strength, elongation, phase transformation, and precipitation; at low temperatures, due to the weakened mobility of atoms, the limitation of dislocation slippage is limited. Plastic deformation may occur from plastic to brittle, that is, low-temperature brittleness. However, in complex materials, other deformation mechanisms become competitive at low temperatures and become an alternative mechanism for deformation. A high-entropy alloy (HEA) is a representative example. At low temperatures, high-entropy alloys exhibit unique mechanical properties due to the combined action of slip and twinning mechanisms. In the process of plastic deformation, it is of considerable significance to find out how various deformation mechanisms compete or cooperate.
The City University of Hong Kong team used in-situ neutron diffraction to discover the interaction of multiple deformation mechanisms in the high-entropy alloy at ultra-low temperature, firstly dislocation slip, then stacking faults and twins, and finally transformed into zigzag deformation into Non-uniform deformation.
High-entropy alloys are an exciting class of structural materials composed of a variety of primary elements. They have excellent strength, ductile combination, fracture toughness, corrosion resistance, and hydrogen embrittlement. They also have excellent stability against radiation damage and are therefore considered potential materials for advanced reactor applications. Despite the complex chemical process, high-entropy alloys can form single-phase robust solutions through a very simple lattice. For example, CrMnFeCoNi, also known as Cantor alloy, has a face-centered cubic (fcc) structure.
At room temperature, the deformation of the ternary CrMnFeCoNi and quaternary CrFeCoNi alloys is mainly dislocation slip. At 1000 K, the primary deformation mechanism is diffusion-controlled dislocation creep. At the temperature of liquid nitrogen, the strength and flexibility of CrMnFeCoNi are improved. In addition to dislocation slip, the activation of twins is also considered to be the leading cause of abnormal ductility. Other mechanisms may also work, especially when the temperature is further reduced. At shallow temperatures, such as the temperature of liquid helium, zigzag deformations are observed, which usually deteriorates ductility. At the same time, different deformation mechanisms are sensitively dependent on composition and microstructure.
In the case of multiple deformation mechanisms coexisting, a key question is at what stage each deformation mechanism appears and their impact on the hardening behavior. For this reason, in-situ observation is essential to determine the activation of different deformation mechanisms and to understand their interaction in subsequent deformation processes. Especially for phase transformation deformation, the effect of phase transformation on strain can only be observed by in-situ study at the test temperature.
Here, the researchers conducted in-situ neutron diffraction measurements on three representative fcc high-entropy alloys, namely CrMnFeCoNi, CrFeCoNi, and CrCoNi. They all show a multi-stage deformation process. Quantitative analysis of experimental data shows that stacking faults play a crucial role in bridging dislocation slippage and sawtooth. If there is no stacking fault, the alloy will slide directly from the dislocation to the diagonal. Like other low-temperature alloys, it will fail early, and the flexibility will decrease. The CrMnFeCoNi alloy studied in this paper has a strength of about 2.5GPa and plasticity of about 62% at a low temperature of 15K!
Two factors cause the deformation of stacking faults. First, the alloy is still in the fcc phase at 15 K, which is consistent with the neutron diffraction data of all samples. The change of stacking fault energy largely depends on the short-term chemical order, and can even become dependent on the local configuration. The fcc phase may be kinetically stabilized at low temperatures. Second, the stacking fault can be small enough so that the stacking fault can start a step immediately before the sawtooth. The first-principles calculation research shows that the temperature dependence of stacking fault energy is closely related to entropy, including configuration entropy, vibration entropy, and magnetic entropy.
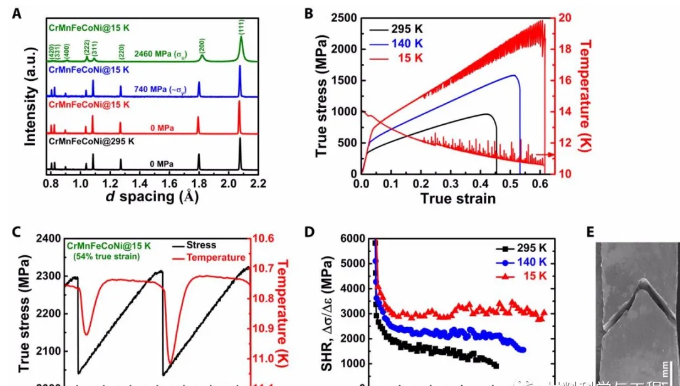
Fig.1 Crystal structure and deformation behavior of CrMnFeCoNi alloy at low temperature
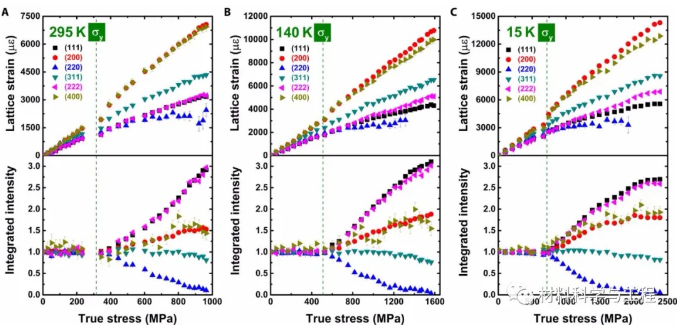
Figure 2 Evolution of lattice strain and texture during deformation
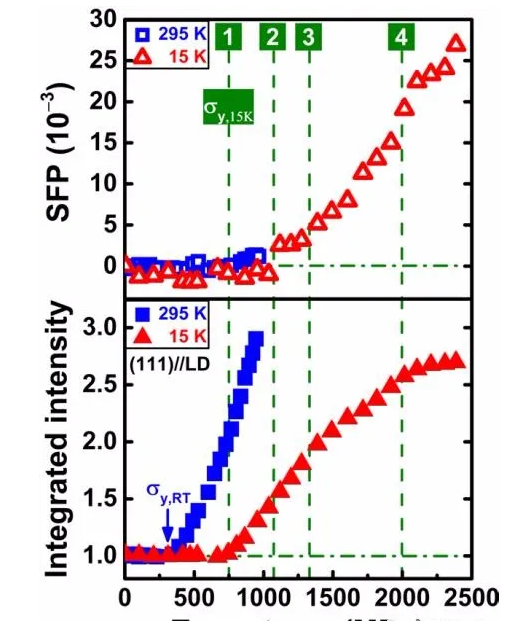
Figure 3 Deformation path of CrMnFeCoNi HEA at 15 K
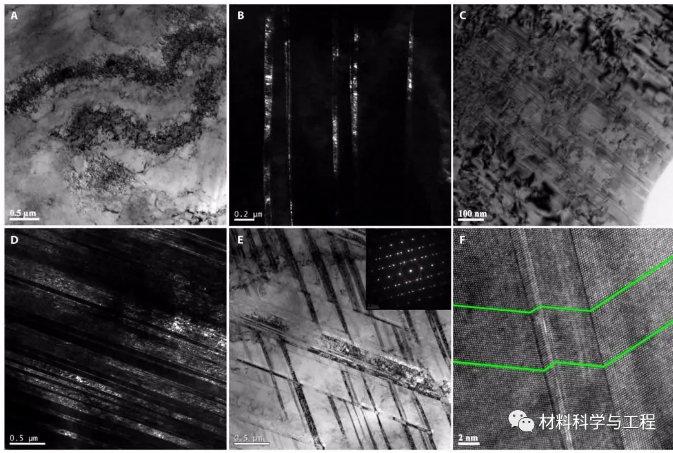
Figure 4 SEM observation results of broken samples
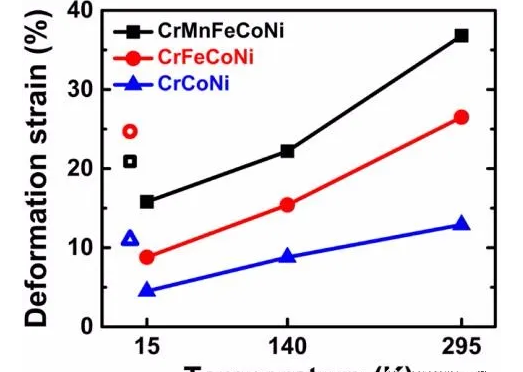
Figure 5 Deformation of fcc high-entropy alloy
This shows that by studying the deformation behavior of high-entropy alloys at ultra-low temperatures, the interaction and competition between different deformation mechanisms are clarified. Research shows that the unique combination of complex chemical composition and simple crystal structure may be the key to the design of new materials for low-temperature applications. In low-temperature applications, various competitive mechanisms can work together to produce high-strength and high-ductility materials.




